RESEARCH ARTICLE
Development of Dry Powder Inhaler Containing Prothionamide-PLGA Nanoparticles Optimized Through Statistical Design: In-vivo Study
Sujit K. Debnath1, *, Saisivam Srinivasan2, Monalisha Debnath1
Article Information
Identifiers and Pagination:
Year: 2017Volume: 4
First Page: 30
Last Page: 40
Publisher Id: TONMJ-4-30
DOI: 10.2174/1875933501704010030
Article History:
Received Date: 23/06/2017Revision Received Date: 28/09/2017
Acceptance Date: 13/10/2017
Electronic publication date: 01/11/2017
Collection year: 2017
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective:
The objective of the present work was to formulate Prothionamide (PTH) nanoparticles using Poly lactic co-glycolic acid (PLGA), optimized by Box-Behnken Design and further modification to dry powder inhaler followed by in-vivo study.
Methods:
Poly-lactic co-gycolic acid (PLGA), a biodegradable polymer was used to coat Prothionamide by solvent evaporation technique. Formulation was optimized using Box-Behnken Design. Response surface curve and desirability factors helped in the selection of optimum formulation of PTH nanoparticles. Dry powder inhaler was prepared by adding inhalable grade lactose to optimize PTH nanoparticles. Mass median aerodynamic diameter (MMAD) was carried out using Andersen Cascade Impactor (ACI) to demonstrate its suitability in the pulmonary administration. In-vitro drug release of dry powder inhaler was carried out in simulated lungs fluid. Correlation in-vitro to in-vivo was established after performing animal experiment.
Results:
FTIR study reveals no chemical interaction between PTH, lactose and PLGA as the principle peaks was retained with same intensity in the physical mixture. Scanning electron microscope showed the spherical shape and aerodynamic particle size was found to be 1.69µm. Drug release study showed initial burst release followed by zero order release. In-vivo model confirmed the presence of PTH after 24h. Aerodynamic particle size and the release profile revealed the suitability of PTH loaded nanoparticles containing dry powder inhaler for the pulmonary administration.
Conclusion:
Prepared DPI containing PTH nanoparticles can improve in the management of tuberculosis by increasing PTH residency in the lungs tissue for prolong period of time.
1. INTRODUCTION
Tuberculosis (TB) is a common and often deadly infectious disease caused mostly due to Mycobacterium tuberculosis. It is an air borne disease, therefore lungs are inflated more recurrently [1]. The main downside of conventional TB treatment is the progress of multiple drug resistance (MDR), resulting in high dose requirements follow by toxicity risk [2]. The thioamide drugs Prothionamide (PTH) is clinically effective in the treatment of some mycobacterial infections. It is bactericidal and recurrently prescribes as second-line drug, particularly in patients with MDR-TB. Due to short half life (2h) and protein binding (15%), it necessitates repeated administration. In addition, less site specificity is demonstrated by PTH and thus the amount of the drug attainment to lungs tissue becomes inadequate [3]. Hence, pulmonary route- an alternate, persuasive and noninvasive route for local delivery of macromolecules was selected for delivering PTH in the form of dry powder inhaler. This route of administration provides large surface area, thin epithelial barrier, avoids first-pass metabolism and high blood flow. In fact, sphere-shaped particles less than 10 μm in diameter can be inhaled. Whereas, particles less than 2.5 μ can accomplish the alveoli [4]. Over the past couple of decades, the field of drug delivery has been revolutionized with the introduction of nanoparticles, wherein these particles act as excellent carriers for drugs to target cells or tissues [5]. Biodegradable polymers such as poly(D,L-lactic acid) (PLA), poly D,L-lactic-co-glycolic acid (PLGA), and poly-ε-caprolactone (PCL) and their copolymers di-blocked or multi blocked with poly ethylene glycol (PEG) have been generally used to form polymeric nanoparticles (NPs) to encapsulate a variety of therapeutic compounds [6]. Safety coupled with commercial availability of PLGA in several monomer ratios and molecular weights have made it a preferred material for wide variety of drugs ranging from small-molecular-weight therapeutic agent to peptide hormones, antibiotics and chemotherapeutic drugs [7]. Solubility of the active ingredient is the foremost pharmaceutical disquiet in developing of novel drug delivery systems [8]. PLGA based nanoparticulate system is one of the most triumphant and fascinating colloidal systems, which successfully delivers the poorly soluble drug. PLGA nanoparticles shield the therapeutic agents, boost stability and used for controlled delivery with improved pharmacokinetic and pharmacodynamic profile [9]. PLGA degrades by hydrolysis, which leads to formation of lactic acid and glycolic acid that are rapidly clear from the body as by-products [3]. The ester linkages react in the presence of water, and rupture away from each other. The rate at which the polymer breaks down is reliant on the ratio of the monomer used in the production [10]. The drug loading is one of the key issues in the process of such an encapsulation. The time of drug intake could be minimized if high drug loading carriers are administered [11]. Both Prothionamide and PLGA are hydrophobic in nature and hence encapsulation is easy. In the present study, PLGA (75:25) was used in the formulation of PTH nanoparticles to decrease the dosing frequency and increase the PTH residency time in lungs. In the optimization of nanoparticles, statistical design employed to meet the industrial requirement. Optimized nanoparticles further modified into dry powder inhaler to make suitable for pulmonary administration and targeting PTH to lungs.
2. MATERIALS AND METHODS
2.1. Materials
Prothionamide & Poly Vinyl Alcohol (PVA) were procured from Yarrow Chem, Mumbai. PLGA 75:25 (Resomer RG 755 S) was obtained as a gift sample from Evonik Industries, Darmstadt, Germany. Lactose anhydrous for inhalation (40M) was obtained as a gift sample of Kerry ingredients, Rothschild, USA. All the other chemicals and reagents were of analytical grade from Merck Millipore, Mumbai.
2.2. Compatibility Study
FTIR analysis was performed to study the chemical interaction between Prothionamide, PLGA (75:25) and inhalable grade lactose using Perkin Elmer (Massachusetts, USA). FTIR spectra were recorded at room temperature to verify alteration in frequency and intensity of bands of pure drug in the presence of excipients [12]. Two samples were run, one for Prothionamide and another for the physical mixture of Prothionamide, anhydrous lactose and PLGA. The samples were scanned in the IR range from 400 to 4000 cm−1.
2.3. Preparation of PLGA-Prothionamide Nanoparticles
PLGA nanoparticles were prepared by solvent evaporation technique as reported earlier [13]. PLGA and PTH were dissolved in common solvent of Dichloromethane and further transferred to 10ml PVA solution followed by vortexed for 10 min. Micro-emulsion was formed when sonicated for 5min over the ice bath using probe sonicator, Bandelin electronic, Sonoplus. The prepared emulsion kept on magnetic stirrer at room temperature for 2h to evaporate Dichloromethane. The nanoparticles were recovered by ultracentrifugation at 18000 rpm for 25 min, followed by single wash with distilled water. Prepared nanoparticles were freeze-dried using 2% w/v D-mannitol as cryoprotectant [14].
2.3.1. Experimental Design
Particle size with suitable and uniform distribution of particle size is the utmost criteria for controlled drug delivery system. Along with high drug loading, there is also the requirement of delivering less amount of drug in pulmonary delivery. These parameters depend on many factors like polymer amount, surfactant concentration and organic phase volume. Polymer amount increases the particle size as well as entrapment efficiency, whereas surfactant concentration and organic phase volume influences the particle size and charging of particles. From the preliminary trials, a 33 Box-Behnken Design was employed to study the effect of three independent variables, i.e. PLGA amount (X1), PVA concentration in % w/v (X2), and Dichloromethane volume in ml (X3), on three dependent variables like particle size (Y1), percentage drug entrapment (PDE) (Y2), Polydispersibility index (Y3). Effects of independent variables were studied at three different levels (Table 1). Nanoparticles were prepared as per the experiment design matrix generated by the software.
2.3.2. Characterization of Nanoparticles
After centrifugation, un-entrapped PTH in the supernatant was estimated by UV-spectrophotometric method as reported previously [15]. Entrapped drug was calculated by subtracting un-entrapped drug from total drug. Further percentage drug entrapment was calculated from the entrapped drug [3, 4]. Average particle size and Poly Dispersity Index (PDI) of the prepared nanoparticles were determined by laser dynamic light scattering using Zetatrac, Microtac Inc.USA. The PDI value indicates the particle size distribution of nanoparticles in a given sample. Higher value of PDI indicates low homogeneity due to wide distribution of NPs, which results in aggregation of particles. Zeta potential indicates the surface charge on the particles and was measured to determine the stability of nanoparticles in the suspension [16].
2.3.3. Scanning Electron Microscopy (SEM)
The shape and surface morphology of the nanoparticles were examined for the optimized batch by Scanning Electron Microscopy (Karl Zesis with SE detector, EVO-18). The samples were sputter-coated with gold and observed for morphology at an acceleration voltage of 7.0 kV with 10.66KX and highest magnification of 19.99 KX.
2.4. Formulation of Dry Powder Inhaler (DPI) and Characterization
Prothionamide nanoparticles and anhydrous inhalable lactose were mixed manually using geometrical dilution process. Bulk and tapped density were carried out in each stage upon addition of lactose. Optimization of dry power inhaler was carried out based on the excellent flow property. Particle size and zeta potential were carried out to check the stability of nanoparticles in the form of DPI.
2.4.1. Determination of Mean Median Aerodynamic Diameter (MMAD)
Aerodynamic diameter of a particle controls its deposition in pulmonary tract. MMAD represents aerodynamic diameter below which 50% particles remain. MMAD of the optimized dry powder inhaler was determined using an eight stage cascade impactor. Firstly, DPI were passed through the cascade impactor with a flow rate of 28.3 l/min, thereafter calculated the Prothionamide content in each stage by direct measurement of Prothionamide using RP HPLC C18 Column, length-250mm. The mobile phase selected was water: Acetonitrile at 70:30 ratio with a flow rate 1.0ml/min. Absorbance was detected at 290nm. These values were inserted into the MMADCALCULATOR to obtain MMAD and geometric standard deviation (GSD) [17].
2.4.2. In-vitro Release Study
5 mg PTH equivalent DPIs were dispersed in 2ml of Simulated lungs fluid (SLF) of pH 7.4 [18, 19] and subsequently filled in dialysis bag (12,000 molecular cut off). To avoid floating, the drug filled bag was tied over the glass plate and kept at the bottom of the dissolution chamber. In-vitro release study was carried out in 1L SLF of pH 7.4 at 37 ±0.5ºC with a paddle speed of 100 rpm [20]. 10 mL of dissolution fluid was withdrawn each hour up to 24h and replaced with fresh SLF.
2.4.3. Accelerated Stability Study
Accelerated stability study was carried out as per the ICH guideline Q1A (R2). Paraffin tape was used to seal cryoprotectant vials contained freshly prepared DPI of PTH nanoparticles. These vials were kept in stability chamber and maintained at 25±2°C & 60±5% RH. The nanoparticles were analyzed for the period of 6 month [15, 21, 22]. Zeta size, zeta potential, PDI, drug entrapment and drug release were carried out to check the stability of dry powder inhaler of PTH nanoparticles.
2.4.4. In-vivo Study
Animal experiments were performed according to the guidelines of institutional animal ethical committee of Bengal College of Pharmaceutical Sciences and Research, Durgapur, West Bengal with prior approval (Registration No: 1799/PO/ Ere/15/S/CPCSEA under CPCSEA, India). Wistar rats (either sex, 4-6 month old & average weight 200-250g) were used in this study to assess the pharmacokinetic parameters of PTH in the form of DPI. The rats were housed for 12-h light/dark cycle with free availability of food and water. Rats were divided into two groups: one was treated with DPI of Prothionamide nanoparticles; and the other group was treated with pure Prothionamide [23]. Dry powder inhaler delivery device was designed with little modification as described previously [24]. Tongue was gently pulled outside and sprayed the DPI after placing the DPI device in the trachea region. After administration, three separate studies were performed. 1ml blood was withdrawn from the tail vain of rat to check the bio-distribution of PTH at 1, 2, 3, 6, 12 & 24h. After scarification in these specified time interval, lungs were isolated and filled it with 4 ml Phosphate buffer solution (PBS) by injected slowly in fractions to fill the lungs [22]. The fluid was withdrawn very slowly followed by one repetition. To estimate PTH remaining to be released, this fluid was vortexed for 5 min with 1ml of methanol. To check the tissue distribution, lungs and trachea portion were homogenized with 10 ml of PBS solution and 1 ml methanol followed by vortexed for 5min. 30% trichloroacetic acid was added to each sample for deprotenization. After neutralized with sodium bi carbonate, each sample was centrifuged for 10min at 8000rpm. Supernatant containing Prothionamide was estimated by HPLC as described previously. Maximum concentration attained by PTH (Cmax) at specified time (Tmax) and area under curve (AUC) were calculated thereafter.
3. RESULT AND DISCUSSION
Prothionamide-PLGA Nanoparticles were prepared by solvent evaporation method. In this study, a seventeen-run, three factors and three levels Box-Behnken design was employed to construct polynomial models for the optimization process. This design was suitable for investigating the quadratic response surface and constructing a second order polynomial model using Design-Expert software (Trial Version7.1.6, Stat-Ease Inc., MN)(24).
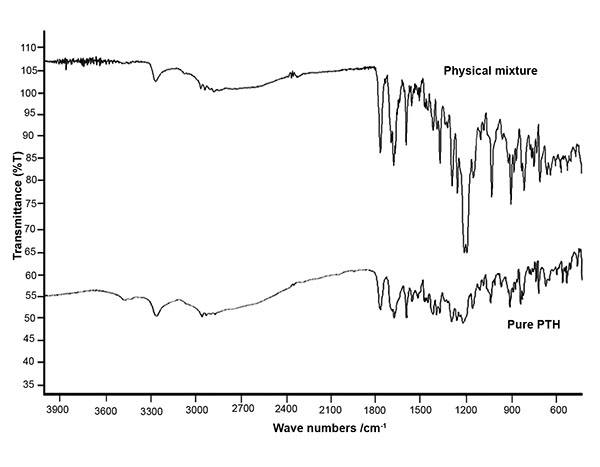 |
Fig. (1). FTIR of pure Prothionamide and physical mixture of Prothionamide, PLGA and lactose. |
3.1. Compatibility Study
Major principle peaks of aromatic stretching, C-N stretching, C=S stretching, =C-H bending were observed for pure Prothionamide at 1592.91, 1211.08, 1024.02 and 900.59 cm-1 respectively. Same peaks were observed in mixture at 1604.48, 1223.13, 1027.50, and 889.0 cm-1 respectively. FTIR spectra (Fig. 1) confirmed the absence of chemical interaction by maintaining the integrity of principle peaks.
3.2. Characterization of Prothionamide Nanoparticles
PLGA nanoparticles containing PTH were prepared by solvent evaporation techniques. Un-entrapment drug was calculated from supernatant after centrifugation using UV-spectrophotometric method. Entrapment efficiency was calculated from it and laid in the range of 55-82%. PDE values were increased with increasing the PLGA amount. The values of particle size, PDE, PDI are shown in Table 2.
3.3. Experimental Design
The design consisted of replicated center points and a set of points lying at the midpoints of each edge of the multidimensional cube, which defined the region of interest used to evaluate the main effects, interaction effects, and quadratic effects of the formulation ingredients, and to optimize the formulation. After filling the data system, suggested quadratic model was followed for further analysis.
3.3.1. Effect of Independent Variables on Particle Size
Polymer amount is known to play an important role in controlling particle size along with release of drug from the matrix. Average particle size was found in the range of 224 to 510nm. The effect of average particle size was explained by the following quadratic equation:
(Y1)= + 299.67 + 20.87X1 - 22.50X2 + 30.13X3 + 13.25X1X2+ 92.50X1X3 - 27.25X2X3 + 20.17X12 - 37.58X22 + 48.17X32.........1
X1, X2 and X3 had the significant effect on the particle size, as the coefficient values were more. Positive effect was seen in X1 and X3 variable due to ‘+ve’ sign. Hence, the average particle size of nanoparticles was increasing with increasing the PLGA amount. Our results are in accordance with those observed by other authors [16, 25]. Mainardes et al. (2005) reported particles size increased with rising the volume of Dichloromethane, but here particle size increased with increasing Dichloromethane volume [13]. On the other side, negative value of factor X2 signifies that the particle size is indirectly proportional with the increasing PVA concentration. With decrease in PVA concentration, the particle size of the NPs increased [16].
3.3.2. Effect of Independent Variables on Entrapment Efficiency
Percentage drug entrapment (PDE) of prepared nanoparticles was found to be in the range of 55 to 82%. The effect of average particle size was explained by the following quadratic equation:
(Y2)= +72.67 + 2.13X1 - 6.63X2- 3.00X3 + 0.75X1X2- 4.00X1X3-6.00X2X3 - 0.71X12- 3.21X22 + 1.04X32 ........................2
| Independent variables / Factor | Levels | ||
|---|---|---|---|
| -1 | 0 | 1 | |
| X1 = PLGA amount | 25 | 75 | 125 |
| X2 = PVA Concentration (%w/v) | 0.25 | 0.75 | 1.25 |
| X3 = Dichloromethane volume (mL) | 2 | 4 | 6 |
| Dependent variables / Response | Constraints | ||
| Y1 = Particle size (nm) | Minimum | ||
| Y2 = Percentage Drug entrapment (%w/w) | Maximum | ||
| Y3= Poly Dispersibility Index | Minimum | ||
| Run | Factor X1 | Factor X2 | Factor X3 | Response Y1 | Response Y2 | Response Y3 |
|---|---|---|---|---|---|---|
| 1 | 125 | 1.25 | 4 | 295 | 64 | 0.607 |
| 2 | 125 | 0.25 | 4 | 314 | 77 | 1.363 |
| 3 | 75 | 1.25 | 2 | 285 | 74 | 1.696 |
| 4 | 125 | 0.75 | 6 | 510 | 69 | 0.964 |
| 5 | 125 | 0.75 | 2 | 265 | 82 | 1.456 |
| 6 | 25 | 0.25 | 4 | 296 | 75 | 2.248 |
| 7 | 75 | 0.25 | 2 | 275 | 74 | 2.095 |
| 8 | 75 | 0.25 | 6 | 390 | 79 | 0.816 |
| 9 | 25 | 0.75 | 6 | 286 | 72 | 1.192 |
| 10 | 75 | 0.75 | 4 | 294 | 71 | 0.652 |
| 11 | 25 | 1.25 | 4 | 224 | 59 | 1.787 |
| 12 | 75 | 0.75 | 4 | 297 | 74 | 1.547 |
| 13 | 75 | 0.75 | 4 | 308 | 73 | 0.827 |
| 14 | 25 | 0.75 | 2 | 411 | 69 | 0.715 |
| 15 | 75 | 1.25 | 6 | 291 | 55 | 1.125 |
| Formulation code | Nanoparticles: Anhydrous lactose | Angle of repose a | Carr’s indexa | Housner ratio a |
|---|---|---|---|---|
| DPI 1 | 1.0:0.5 | 33.68 ± 1.34 | 11.12 ± 0.41 | 1.13 ± 0.001 |
| DPI 2 | 1.0:1.0 | 27.41 ± 0.73 | 8.13 ± 1.92 | 1.09 ± 0.02 |
| DPI 3 | 1.0:1.5 | 32.86 ± 2.38 | 12.08 ± 1.81 | 1.14 ± 0.02 |
| DPI 4 | 1.0:2.0 | 34.98 ± 0.41 | 13.54 ± 1.80 | 1.16 ± 0.02 |
| a Values are mean ± standard deviation | ||||
| Time point (month) | Particle size (nm)a | PDIa | Zeta potentiala | % Drug Entrapmenta | % drug release in 24ha |
|---|---|---|---|---|---|
| 0 | 323.3 ± 10 | 0.321 ± 0.13 | 6.11 ± 0.11 | 80.13 ± 0.11 | 42.51 ± 1.5 |
| 1.5 | 323.8 ± 09 | 0.319 ± 0.21 | 6.19 ± 2.7 | 80.13 ± 0.09 | 43.15 ± 0.9 |
| 3 | 326.1 ± 05 | 0.317 ± 0.32 | 5.9 ± 3.5 | 80.12 ± 0.12 | 43.89 ± 3.2 |
| 6 | 331.4 ± 12 | 0.319 ± 0.47 | 6.5 ± 2.1 | 80.12 ± 0.16 | 48.32 ± 6.4 |
| a Values are mean ± standard deviation | |||||
| Time (h) | Percentage drug release | Concentration of PTH in lungs (µg/mL) | AUC of Pulmonary pharmacokinetic (µgh/mL) | Concentration of PTH in blood (µg/mL) | AUC of plasma pharmacokinetic (µgh/mL) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PTH nano.a | Pure PTH a | PTH nano. a | Pure PTH a | PTH nano. a | Pure PTH a | PTH nano. a | Pure PTH a | PTH nano. a | Pure PTH a | |
| 1 | 19.77 ± 0.89 | 38.51 ± 0.59 | 2.31 ± 0.03 | 4.56 ± 0.31 | 15.79±0.20 | 10.48 ± 2.31 | 0.09 ± 0.005 | 0.42 ± 0.03 | 1.16 ± 0.02 | 0.728 ± 0.01 |
| 2 | 24.65 ± 0.47 | 67.62 ± 2.95 | 2.07 ± 0.01 | 2.05 ± 0.09 | 0.096 ± 0.0003 | 0.14 ± 0.01 | ||||
| 3 | 26.38 ± 1.32 | 91.41 ± 1.22 | 2.03 ± 0.01 | 0.72 ± 0.06 | 0.079 ± 0.005 | 0.08 ± 0.01 | ||||
| 6 | 28.67± 0.69 | 99.84 ± 0.08 | 0.65 ± 0.04 | 0.18 ± 0.03 | 0.074 ± 0.003 | 0.001 ± 0.001 | ||||
| 12 | 33.02 ± 1.04 | --- | 0.28 ± 0.03 | -- | 0.028 ± 0.005 | --- | ||||
| 24 | 40.36 ± 1.47 | --- | 0.22 ± 0.02 | --- | 0.025 ± 0.001 | --- | ||||
| a Values are mean ± standard deviation | ||||||||||
There was less influence of X1, X2 & X3 on the PDE value due to low coefficient value. Influence of X2 on PDE was more in comparison to X3 and X1. Here, percentage drug entrapment was increased by increasing the PLGA amount (X1) and decreasing the PVA concentration (X2) and organic phase volume (X3).
3.3.3. Effect of Independent Variables on Polydispersibllity Index
Polydispersibility Index or PDI (Y3) was found in the range of 0.607 to 2.248. Correlation tried to establish the relation between PDI with X1, X2 and X3. The value of zeta potential of all the batches was found to be within 5mV. Particle aggregation is less likely to occur for charged particles (i.e. high zeta potential) [26]. In this range, particle has more tendency to aggregate resulting in wide distribution of particle size. Hence, no significant influences were established between independent variables with polydispersibiliy index.
3.3.4. Search for Optimum Nanoparticles of Prothionamide
All the measured responses that may affect the quality of the product were taken into consideration during the optimization [27]. Particle size and PDE were taken under consideration for the selection of optimized batch. Formulation was optimization with set criteria of maximum entrapment efficiency and minimum particle size, followed by higher disability factor. Design suggested that optimized formulation contained 125mg of PLGA, 0.36%w/v solution of PVA and 2ml of DCM. Nanoparticles prepared using this composition and found the particle size of 310 ± 12 nm, PDE 80.08 ± 0.26% and PDI of 0.386 ± 0.13.
3.4. SEM Analysis of System Generated Optimized Nanoparticles
SEM images (Figs. 2a and b) showed that the optimized Prothionamide nanoparticles were spherical in shape. Most of the particle size was of 205.6 nm, which are suitable for the inhalation formulation.
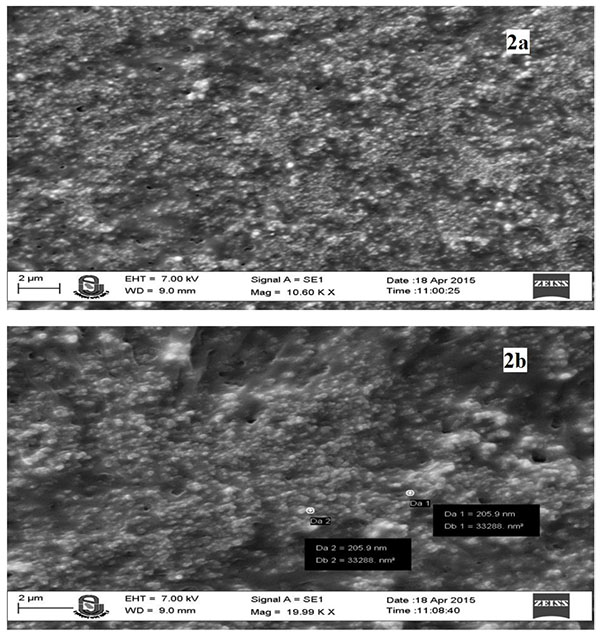 |
Fig. (2). SEM image of optimized freeze dried nanoparticles at 10.66 KX (2a) and 19.99KX (2b). |
3.5. Formulation and Characterization of Dry Powder Inhaler
Different proportion of anhydrous inhalable grade lactose was added to the optimized Prothionamide nanoparticles by physical geometric mixture. In each addition, angle of repose, carr’s index, hausner ratio was determined (Table 3). Upon addition of anhydrous lactose, these values were decreased initially but increased afterward. DPI 2 showed excellent flow property in 1:1 proportion of Prothionamide nanoparticles and lactose.
3.5.1. Determination of MMAD and Geometric Standard Deviation
The size and shape of particles are the main parameters that determine their deposition in the different parts of lungs. Aerodynamic particle size in range of 0.5-5 µm can reach to the small airways and alveoli [28]. Prepared DPI of Prothionamide nanoparticles aerodynamic particle size was 1.69 µm and geometric standard deviation was 1.95. Maximum fine particle fraction, extra fine particle fraction and emitted dose were found as 76.88%, 23.88% and 91.46% respectively. This signifies that the prepared DPI can reach deeply to lungs.
3.5.2. In-vitro Release Study
The optimized Prothionamide nanoparticles (OP Batch) loaded DPI was subjected for in-vitro drug release behavior. SLF was used as dissolution medium for evaluating the release pattern of Prothionamide from PLGA nanoparticles. The optimized Prothionamide-PLGA nanoparticles showed initial burst release of 20% and then showed sustained drug release up to 43.52% in 24h (Fig. 3). The initial burst release will help to reach the desired plasma concentration in lungs, and the sustained release will help to maintain the PTH dose for prolong period of time. The in-vitro release data was analyzed through zero order, Higuchi, Peppas models and obtained correlation co-efficient (r2) were found to be 0.982, 0.966, and 0.933 respectively. The release best fitted with zero order kinetics.
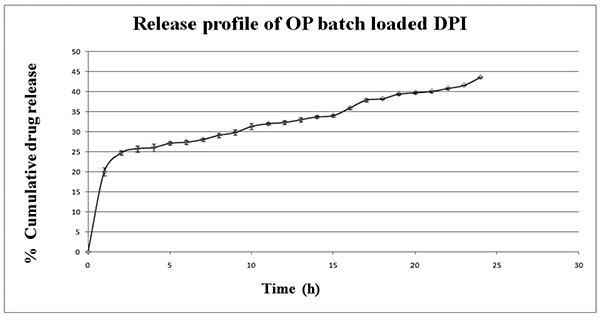 |
Fig. (3). In-vitro release of optimized DPI in simulated lungs fluid at pH 7.4. Data represents mean ± SD (n=3). |
3.5.3. Accelerated Stability Study
Various literatures reported the instability of nanoparticles during storage. 6 month accelerated stability study revealed no clamping or aggregation of particles. Zeta size, PDI, and drug release demonstrated that the conservation of dry powder inhaler was stable during stress testing (Table 4).
3.5.4. In-vivo study
The minimum inhibitory concentration (MIC) of Prothionamide (0.5µg/ml) was obtained for approximately 16h by a regimen of 250–375 mg twice daily [29]. But this dose is never suitable for the pulmonary administration. So, pulmonary dose was calculated based on the human lungs volume (4.341 l) [21]. The prepared DPI of Prothionamide nanoparticles contained two parts: loading and maintenance dose. As both the dose release at a time, corrected dose was calculated as 78 mg for human administration. Dose calculation for rat was carried out with equivalent surface and weight with no observed adverse effect in 7.7mg/kg body weight [22]. Three different studies were carried out to determine percentage drug release in-vivo, lungs tissue distribution and bio-distribution of Prothionamide (Table 5). In contrast to pure PTH administration, PTH nanoparticles gave sustained release more than 24h, whereas pure PTH gave release 99.84±0.08 in 6h. AUC increased significantly when PTH was given in the form of nanoparticles. As PTH reached very slowly to plasma from PTH nanoparticles, AUC was relatively less 1.16±0.02 µgh/mL in comparison to pure PTH. Cmax was 2.31±0.03 µg/mL for PTH DPI, whereas 4.567 ± 0.31 µg/mL for pure PTH reached at Tmax 1h. DPI of PTH nanoparticles maintained plasma concentration above the MIC for the period of 6h, but pure PTH could be maintained for up to 3h. Concentration attained by PTH nanoparticles 0.22 ± 0.02 µg/mL, which is lower than the MIC. Hence, prepared formulation may be used once every day to maintain the plasma concentration above MIC for 24h. PTH was detected after 24h, when given in the form of nanoparticles, which signified prolong drug residency in lungs.
CONCLUSION
In this study, Prothionamide-PLGA nanoparticles were optimized by statistical Box-Behnken design. Optimization study revealed that the PLGA amount and surfactant concentration had prominent effect on entrapment efficiency and particle size. Aerodynamic particle size of the DPI containing PTH nanoparticles was favorable for the effective delivery in pulmonary administration. In-vitro zero order release profile of DPI also signifies the residence of Prothionamide for prolong period of time in lungs, which has been confirmed after animal experiment. Animal studies successfully correlate with the in-vitro release profile in simulated lungs fluid. Prepared DPI containing PTH nanoparticles could improve the effectiveness of the treatment by increasing PTH concentration in the lungs tissue with single dose administration daily. Future prospect is to check the level of PTH concentration above MIC for 24h by daily dose administration.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial, or profit sectors.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Animal used in this work with permission of institutional animal ethical committee of Bengal College of Pharmaceutical Sciences and Research, Durgapur, West Bengal with prior approval (Registration No: 1799/PO/ Ere/15/S/CPCSEA under CPCSEA, India).
HUMAN AND ANIMAL RIGHTS
The study was in accordance with the standards set forth in the 8th Edition of Guide for the Care Use of Laboratory Animals (http:// grants.nih.gov/grants/olaw/Guide-for-the-care-and-use-of-laboratory-animals.pdf) published by the National Academy of Sciences, The National Academies Press, Washington DC, United States of America.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLDEGEMENTS
Authors would like to acknowledge the Department of Seed Science & Technology, Agriculture University, Junagadh for providing free service of Remi centrifugation machine and Bengal College of Pharmaceutical Sciences and Research, Durgapur for providing excellent infrastructure to conduct animal study. Authors are also thankful for the cordial support of Dr. Jayrajsinh Sarvaiya (Assistant Professor) for pursuing the research work.
REFERENCES
| [1] | Bijev A, Georgieva M. The development of new tuberculostatics addressing the return of tuberculosis: Current status and trends. J Univ Chem Technol Metall 2010; 45(2): 111-26. |
| [2] | Kumar N, Kumar P, Kumar M, Kumar R. Nanotechnology: A focus on treatment of tuberculosis. Int J Drug Deliv 2011; 3(1): 25-42. |
| [3] | Jain DS, Athawale RB, Bajaj AN, et al. Unraveling the cytotoxic potential of Temozolomide loaded into PLGA nanoparticles. DARU J Pharm Sci 2014; 22(1): 18. |
| [4] | Pirooznia N, Hasannia S, Lotfi AS, Ghanei M. Encapsulation of alpha-1 antitrypsin in PLGA nanoparticles: in vitro characterization as an effective aerosol formulation in pulmonary diseases. J Nanobiotechnology 2012; 10(20): 1-15. |
| [5] | Vij N, Min T, Marasigan R, et al. Development of PEGylated PLGA nanoparticle for controlled and sustained drug delivery in cystic fibrosis. J Nanobiotechnol 2010; 8(22): 1-18. |
| [6] | Chan JM, Zhang L, Yuet KP, et al. PLGA-lecithin-PEG core-shell nanoparticles for controlled drug delivery. Biomaterials 2009; 30(8): 1627-34. |
| [7] | Sameni J, Bukhari NI, Azlan NA, Julianto T, Majeed AB. The Effect of Preparation Parameters on the Size and Morphology of PLGA-Based Nanoparticles 2009; 700-4. |
| [8] | Averineni RK, Shavi GV, Gurram AK, et al. PLGA 50:50 nanoparticles of paclitaxel: Development, in vitro anti-tumor activity in BT-549 cells and in vivo evaluation. Bull Mater Sci 2012; 35(3): 319-26. |
| [9] | Manoochehri S, Darvishi B, Kamalinia G, et al. Surface modification of PLGA nanoparticles via human serum albumin conjugation for controlled delivery of docetaxel. Daru J Pharm Sci 2013; 21(58): 1-10. |
| [10] | Hamilla SM, Michael H, Ashley M. Microparticle drug delivery syringe. 2011 IEEE 37th Annual Northeast Bioengineering Conference 2011; 1-2. |
| [11] | Wang Y, Li P, Kong L. Formulation optimization for high drug loading colonic drug delivery carrier 2010; 1686-9. |
| [12] | Kambham V, Kothapalli Bonnoth C. Development of stavudine sustained release tablets: In-vitro studies. Futur J Pharm Sci [Internet] 2016; 2(2): 37-42. |
| [13] | Mainardes RM, Evangelista RC. PLGA nanoparticles containing praziquantel: Effect of formulation variables on size distribution. Int J Pharm 2005; 290(1-2): 137-44. |
| [14] | Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv Drug Deliv Rev 2006; 58(15): 1688-713. |
| [15] | Debnath SK, Saisivam S, Dash DK, Debnath M. Development and validation of UV-spectrophotometric methods for quantitative estimation of Prothionamide in pure and pharmaceutical dosage forms. Int Curr Pharm J 2015; 4(7): 402-4. |
| [16] | Sharma D, Maheshwari D, Philip G, et al. Formulation and optimization of polymeric nanoparticles for intranasal delivery of Lorazepam using Box-Behnken design: In vitro and in vivo evaluation. Biomed Res Int 2014; 1-14. |
| [17] | Sinha B, Mukherjee B. Development of an inhalation chamber and a dry powder inhaler device for administration of pulmonary medication in animal model. Drug Dev Ind Pharm 2012; 38(2): 171-9. |
| [18] | Marques MR, Loebenberg R, Almukainzi M. Simulated biological fluids with possible application in dissolution testing. Dissolut Technol 2011; 18(3): 15-28. |
| [19] | Debnath SK, Saisivam S, Omri A. PLGA ethionamide nanoparticles for pulmonary delivery: Development and in vivo evaluation of dry powder inhaler. J Pharm Biomed Anal 2017; 145: 854-9. [Internet]. |
| [20] | May S, Jensen B, Wolkenhauer M, Schneider M, Lehr CM. Dissolution techniques for in vitro testing of dry powders for inhalation. Pharm Res 2012; 29(8): 2157-66. |
| [21] | Fernandes CA, Vanbever R. Preclinical models for pulmonary drug delivery. Expert Opin Drug Deliv 2009; 6(11): 1231-45. |
| [22] | Chougule M, Padhi B, Misra A. Nano-liposomal dry powder inhaler of tacrolimus: preparation, characterization, and pulmonary pharmacokinetics. Int J Nanomedicine 2007; 2(4): 675-88. |
| [23] | Guidance for industry. Estimating the maximun safe starting close in initial clinical trial for therapeutics in adult health volunteers.. 2005. Available at: http://www.fda.gov/downloads/Drugs/Guidance/UCM078932.pdf |
| [24] | Okuda T, Suzuki Y, Kobayashi Y, et al. Development of biodegradable polycation-based inhalable dry gene powders by spray freeze drying. Pharmaceutics 2015; 7(3): 233-54. |
| [25] | Tripathi A, Gupta R, Saraf SA. PLGA nanoparticles of anti tubercular drug: Drug loading and release studies of a water in soluble drug. Int J Pharmatech Res 2010; 2(3): 2116-23. |
| [26] | Kharia AA, Singhai AK, Verma R. Formulation and evaluation of polymeric nanoparticles of an antiviral drug for gastroretention. Int J Pharm Sci Nanotechnol 2012; 4(4): 1557-62. |
| [27] | Pandya VM, Patel JK, Patel DJ. Formulation and optimization of nanosuspensions for enhancing Simvastatin dissolution using central composite design. Dissolut Technol 2011; 18(3): 40-5. |
| [28] | Fernández Tena A, Casan Clarà P. Deposition of inhaled particles in the lungs. Arch Bronconeumol 2012; 48(7): 240-6. |
| [29] | Lee HW, Kim DW, Park JH, et al. Pharmacokinetics of prothionamide in patients with multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2009; 13(9): 1161-6. |




